Careful and Proactive Planning of Packaging and Distribution Strategy Saves Time and Cost
5 Minute Read

Once a clinical trial is in progress, a variety of causes, such as fluctuating enrollment or emerging clinical safety or PK data, may indicate a need to adjust the clinical trial drug product or packaging before the next dosing period. Sponsors and biotech clients often worry that changing the plan for clinical trial materials may result in delays and higher costs.
At Societal CDMO™, regardless of the size of your organization, whether your study is large-scale or niche, we provide focused, flexible, responsive collaboration and pivot quickly to provide the clinical trial materials you need. Our staff partners closely with you, acting as an extension of your business and quickly reacting to changes to help ensure your study stays on track.
Supply chain expertise, thoughtful and proactive up-front planning of your supply chain strategy, combined with flexible packaging and distribution solutions, ensure that you receive correctly packaged and labeled clinical trial materials when and where you need them. Industry-leading regulatory advice helps you understand what you need to file — and what you don’t.
A Flexible and Responsive Packaging and Distribution Strategy Is Required for a Successful Adaptive Trial
Societal offers a range of clinical trial materials and services, spanning manufacturing, stability testing, and comparator procurement to packaging, labeling, storage, distribution, and returns. To complement these activities is our dedicated project management and clinical supply chain management staff who will take a broad view of your trial to design a supply chain strategy that can include bright stocking and material pooling as appropriate, ideal for adaptive trials.
This efficient option allows you the flexibility to respond to ever-changing clinical trial demands and enrollment challenges. By pooling bright stock clinical trial material, we can package, label, and ship clinical supply material as required. This service is ideally suited for adaptive trial design and other circumstances in which supply chain speed and flexibility are essential.
Large, conventional clinical supply material packaging campaigns have become unsustainable. The days of overpackaging 50% to 100% to mitigate potential stockouts are over. While this degree of waste was never ideal, it is unacceptable in trials and indications involving hard-to-manufacture, costly medicines. A flexible packaging and distribution strategy requires less bulk drug at the initiation of a trial and decreases costs through the course of your trial.
Benefits of Robust Supply Chain Design
- Speedy, agile response
- Reduced early drug development costs; shorter timelines
- Reduced waste
- Lower storage and inventory fees
- Enhanced adaptive trial capability
- Facilitates patient-centric trials
Distribution Solutions
The industry trend is that more and more clinical trials will become virtual. Patients who are in remote locations, homebound, ill, or potentially infected with a contagious virus prefer not to travel to the clinic for an immediate medical need, let alone to participate in a clinical trial. Thus, patient enrollment and retention are a greater challenge than ever for the clinical team developing a new drug.
To assist our customers, Societal’s clinical trial services offer distribution and logistics with help from third-party logistic partners. Our teams collaborate in developing a logistics plan tailored to each protocol.
End-to-End Clinical Trial Supply Chain Management
- Manufacturing — filling, blinding, dosing, over-encapsulation
- Analytical testing — product release, stability studies, comparator testing
- Forecasting — sites, inventory, optimized packaging campaigns
- Comparator procurement
- Packaging — primary and secondary
- Labeling — single panel or booklet
- Storage 15°C-25°C and 35%RH-65%RH
- Distribution and logistics complete support for transport, and distribution and documented chain of custody management
- Returns and destruction
Top-Notch Regulatory Guidance May Help You Avoid Resubmitting to FDA
At Societal, you have uncommon access to real regulatory expertise. Sometimes, changing dosage and the PK profile means reformulating your clinical trial dosage form. By simplifying and isolating changes using a risk-based scientific approach designed to avoid clinical impact, you may be able to construct formulation changes that limit regulatory filing requirements to a chemistry, manufacturing, and controls (CMC) prior approval supplement (PAS) only in accordance with the FDA’s scale-up and post-approval changes (SUPAC) guidance — saving prodigious time and expense.
Integrated Regulatory Support
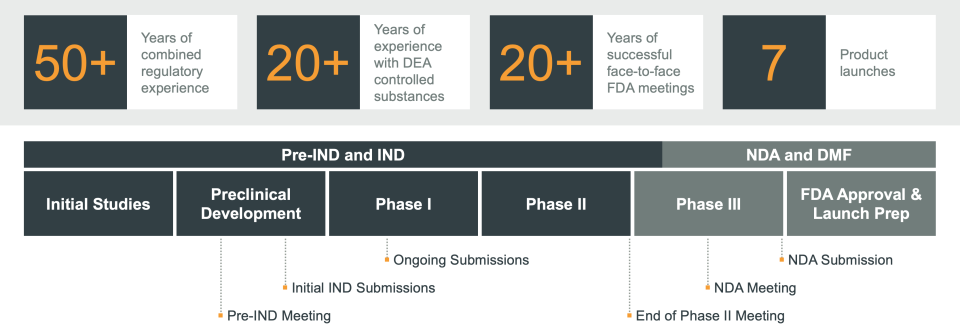
From developing sound adaptive trial designs to providing FDA meeting guidance and preparing submissions, our regulatory experts have the experience you need to streamline your product’s pathway to regulatory approval.

Societal Helps You Deliver New Medicines to Those in Need Faster
Whether you need stand-alone primary and secondary pharmaceutical packaging, or a global supply chain management solution, Societal gives you confidence. We respond to mid-study, evolving needs in clinical trial supply quickly and effectively.
Unlike larger CDMOs where smaller clients may be overlooked, Societal partners closely with you to ensure success. You’ll know the names and faces of not just our business development team, but the operations and quality teams as well. With our client-centric approach, Societal serves as an extension of your business, proactively engaging with you throughout the course of your project.
For maximal productivity in drug development, Societal will maximize your drug candidate’s potential for success by delivering the clinical trial supplies you need when you need them, packaged and labeled to your exact specifications.
