Pros and Cons of Matrix vs. Multiparticulate Formulations
7 Minute Read

For Modified Release (Controlled Release) Oral Solid Dosage Forms
Should you design your modified release (controlled release) oral solid dosage form as a multiparticulate capsule or a matrix tablet? Multiparticulates provide specific benefits for certain applications. However, matrix tablets have really come to dominate the field. Why choose one over another? And what is your best approach to develop a robust product as efficiently as possible?
Consider the Ultimate Goal
The first thing to think about is, “What is the intended patient benefit?” Modified release dosage forms should offer a benefit for patients over and above the immediate release version of the same drug.
Therapeutic benefits might be:
- More consistent therapeutic effect through blood level control
- Extended duration of action
- Reduced side effects from a lower Cmax
- Drug release at or near the intended physiologic site of action
- Reduced food effect
- Better bioavailability
Patient compliance benefits might be:
- Reduced dosage frequency
- Greater convenience, fewer pills to swallow
- Better compliance (less cumbersome dosing schedule reduces likelihood of omission or error)
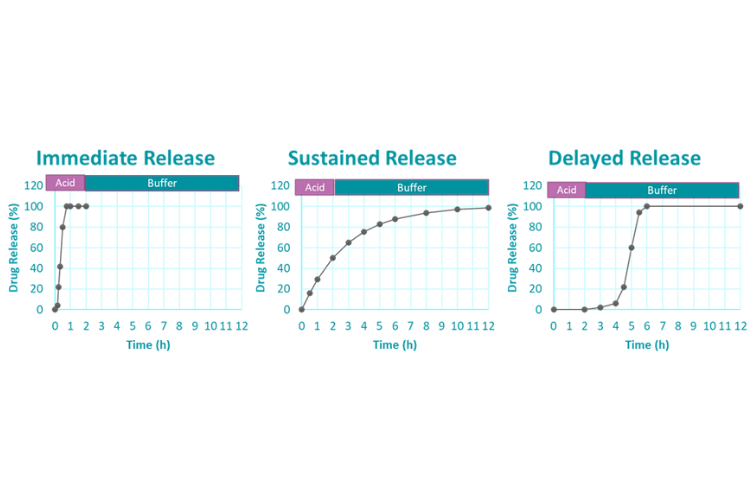
To achieve these goals, modified release dosage forms generally deliver a drug over a prolonged period of time (sustained release), delay drug delivery (delayed release), deliver a drug to a specific gastrointestinal target (pH-dependent pulse release), or a combination of these.
Pharmacokinetic profiles of immediate, sustained, and delayed release dosage forms
These release profile benefits can be achieved and combined by controlling drug release through a variety of mechanisms. Two common mechanisms are diffusion and erosion. The drug can diffuse through either a polymer gel matrix or a polymer coating. Similarly, digestive fluids can erode either a matrix or a coating.
Understand multiparticulate structure and manufacture
The pellets in multiparticulate dosage forms contain an inert core, surrounded by a drug layer, surrounded by a polymer coating that retards the release of the drug. Essentially, you have a multitude of small drug reservoirs with diffusion control or erosion control, depending on the polymer’s properties.
This design has certain implications for how it will benefit the patient — and the company developing it:
- The multiple particles quickly spread out in the GI tract.
- You can put multiple bead populations in a single capsule or tablet.
- You can optimize each of those populations separately, which means that you can separate APIs easily within the same dosage form for either chemical compatibility reasons or to achieve a certain release profile.
- It’s easy to change the dosage strength without affecting the dissolution release profile during development.
- It’s possible to adjust the overall dissolution release profile by adjusting the ratio of the different populations of beads or pellets in the capsule (without having to reformulate the individual pellet populations).

Wurster coater for manufacturing particles
Manufacturing implications are significant: You have to develop the particles or pellets separately. This means:
- Each intermediate pellet requires development, scale-up, process validation, manufacture, and release testing independently of the others and then together as the final dosage form.
- Increased development timeline, cost, and testing burden of the finished product for QC purposes
- The equipment is less common, more expensive, and often produces a smaller batch size than equivalent equipment for manufacturing tablets.
- Examples: Wurster coaters, fluid bed technologies, rotor processors, extruders, spheronizers, and pellet encapsulators
- Manufacturing cycle times tend to be longer than for similar tablet manufacturing.
- A polymer coating process in a Wurster machine at large scale can easily take six to 12 hours or longer.
- All of this leads to a higher cost for your finished dosage form.
- Tech transfer is more difficult.
- Fewer facilities are capable, so you have fewer sites to choose from
- Process is more complicated, expertise is less common
Get to Know Monolithic Matrix Tablet Structure and Manufacture
In monolithic structures, the drug is distributed throughout a polymer matrix in the tablet. The matrix works as one unit, which dissolves, controlling the release. They’re very robust, common, reasonably easy to develop, and inexpensive to make.
Three different types are shown:
- Hydrophilic swellable
- Hydrophobic, non-swellable
- Erodible
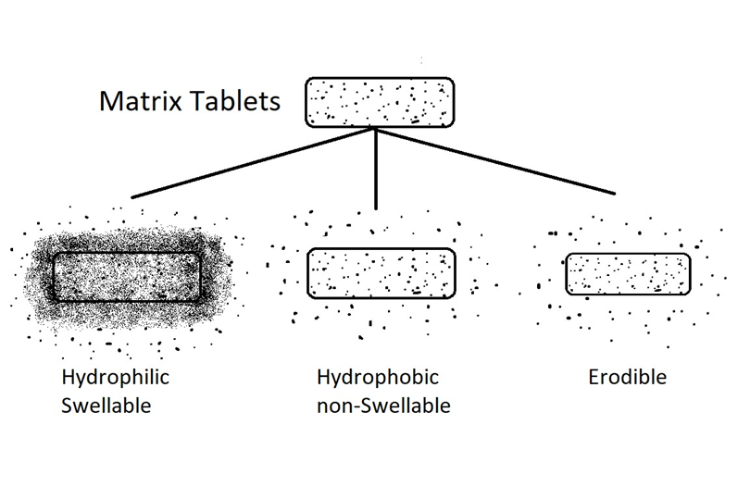
These have different applications. The hydrophilic swellable matrix is the most common. The polymer swells rapidly upon exposure to water, creating a gel that slows drug diffusion. Hydrophobic, non-swellable formulations have a waxy matrix, and they remain intact as a unit. They don’t swell, but the drug leaches out over time. Erodible formulations may, for example, dissolve at a specific pH, exposing and releasing the drug.
The implications of matrix tablet design are a counterpoint to multiparticulate:
- The matrix tablet travels as a single unit through the GI tract.
- It has a single release profile.
- You can’t tune the release profile of two drugs within the same matrix.
- There’s no separation of the APIs unless you do more.
- Add microencapsulation or multiparticulate technology — or make a bilayer tablet.
- Release depends on the geometry and compression of the tablet itself.
- Changing the dosage strength requires reoptimization of either geometry, compression, or formulation to maintain the same dissolution profile.
The manufacture of matrix tablets is really where they stand out:
- Manufacturing is essentially the same as for any immediate release tablet.
- Steps include granulation, blending, compression, and coating
- A very broad manufacturing base with lots of manufacturing capacity is available across the industry.
- Expertise to develop these formulations is less common than it is for immediate release tablets, but more common than for multiparticulates.
- The manufacturing process is similar enough that any manufacturing group making immediate release tablets is also capable of making a modified release tablet.
- You can make higher volumes quickly with faster manufacturing cycle times, lower cost, and easier tech transfers for monolithic matrix tablets than for multiparticulates.
Ask how well these two designs help you reach your goal
We’ve seen that patient benefit may be met through more consistent blood levels or tuning the dissolution profile to fulfill desired pharmacokinetic and pharmacodynamic criteria and more. While either matrix or multiparticulate dosage forms can provide some or all of these benefits, in certain situations, multiparticulates can enable things that are not easily possible with matrix tablets.
For instance, if gastric emptying directly affects the plasma profile because of pH effect on mechanism of release, drug solubility, or drug absorption, multiparticulates may give less patient-to-patient variability.
Whereas it’s easy to change the dose of a multiparticulate by altering fill weight, this is not always possible with a tablet. Creating a biphasic pulsatile release profile in a matrix tablet may only be possible with a low dose. One approach is to create a delayed release core and apply an immediate release coating on the outside. This significantly limits the possible IR portion of the dose because that coating can’t be too thick for suitable processing and product performance. In contrast, pulsatile profiles are easy to achieve with multiparticulates. Just by mixing and matching the same set of pellets, multiparticulates enable you to make some quick changes to the dosage strength or the dissolution profile for your clinical studies.
The Upshot
Matrix tablets are really the go-to if you can use one. They are:
- Cheaper to develop
- Cheaper to manufacture
- Easier to transfer
The downside is that they’re not as flexible. If you have something that is locally irritating, a multiparticulate can spread that dosage across the GI tract. If you have something that’s prone to food effect and highly subject to variability in PK, a multiparticulate may be a much better idea.
Multiparticulates are:
- More versatile
- Generally less variable from patient to patient
- Easier to iterate for clinical studies when you’re trying to fine-tune the PK behavior during your development process
In general, choose matrix unless you have a compelling need for multiparticulate.

